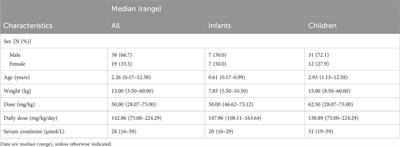
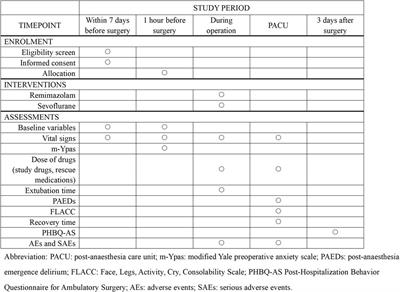
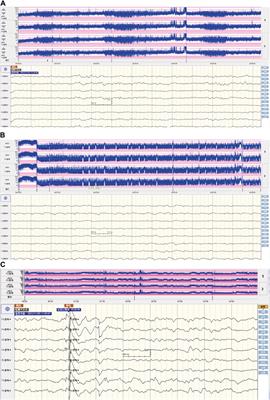
Clinical Trial
Published on 28 Aug 2024
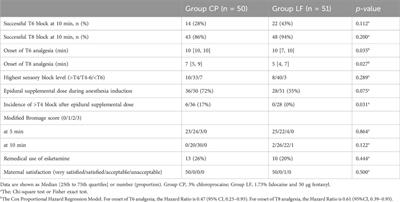
Comparison of lidocaine bicarbonate with fentanyl and chloroprocaine for epidural anesthesia during cesarean section: a randomized, controlled, double-blind clinical trial
in Obstetric and Pediatric Pharmacology
- 1,682 views
- 1 citation